-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
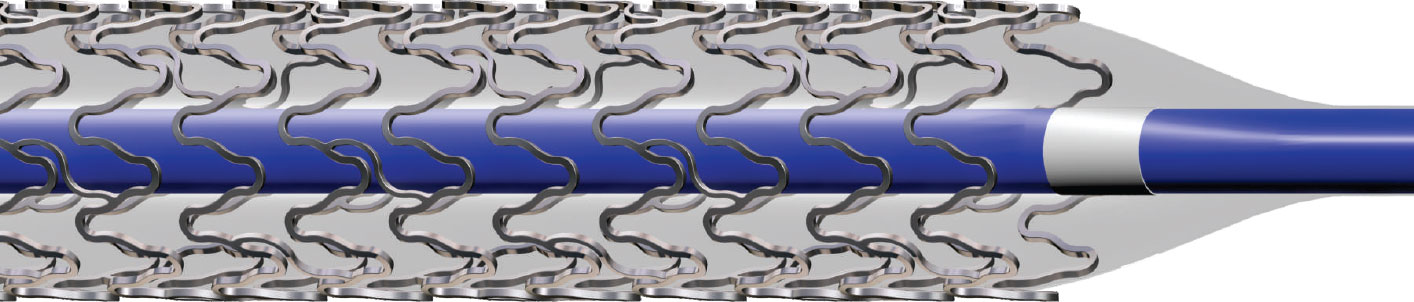
Accuracy of Lesion Coverage: No Foreshortening
Fatigue Resistant: Reduced risk of strut fracture due to bending fatigue
Lower Strut Thickness (150 µm)
Optimal Scaffolding
High Radial Strength
Excellent Deliverability & Conformability
Maximum Circular Unsupported Area (mm)
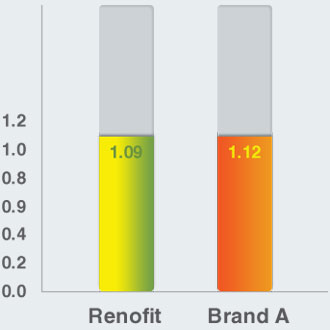
Maximum Circular Unsupported Area (MCUSA)
- Renofit shows comparable MCUSA to that of a leading brand
- Uniform MCUSA across stent design ensures optimal scaffolding
Foreshortening (%) (In Vitro)
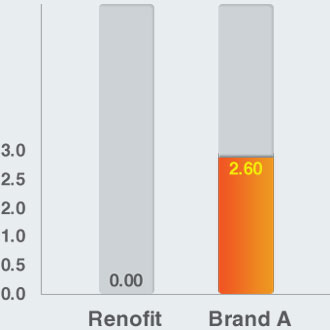
Accuracy of Lesion Coverage
- Renofit depicts no reduction in stent length while Brand A showed foreshortening of 2.6%
Crimped Profile (mm)
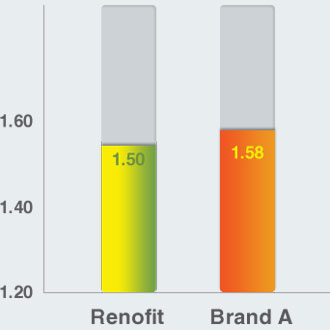
Crimped Profile
- Renofit has lower crimped profile compared to that of a leading brand, enabling itself to be a better option in tight lesions
*Data on File.
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the “Instructions for use” supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As a part of our continuous product development policy we reserve the right to change product specifications without prior notification.
*Test performed by and data on file at Sahajanand Medical Technologies Ltd. Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Sahajanand Medical Technologies Ltd.
Renofit is a trademark of Sahajanand Medical Technologies Ltd. or its affiliates. Specifications are subject to modification, revision and improvement.



