-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
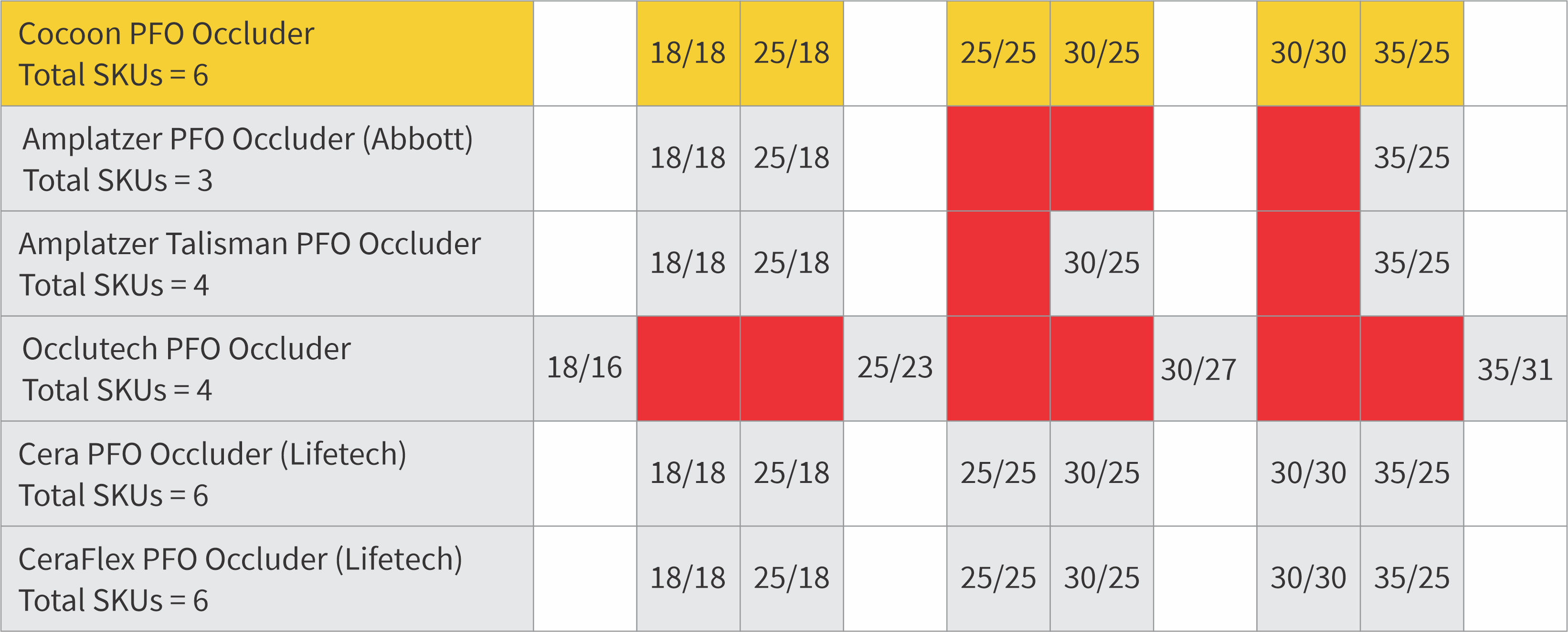
Extensive size matrix so that there is no compromise
6 SKUs (vs 4 SKUs of competitor)
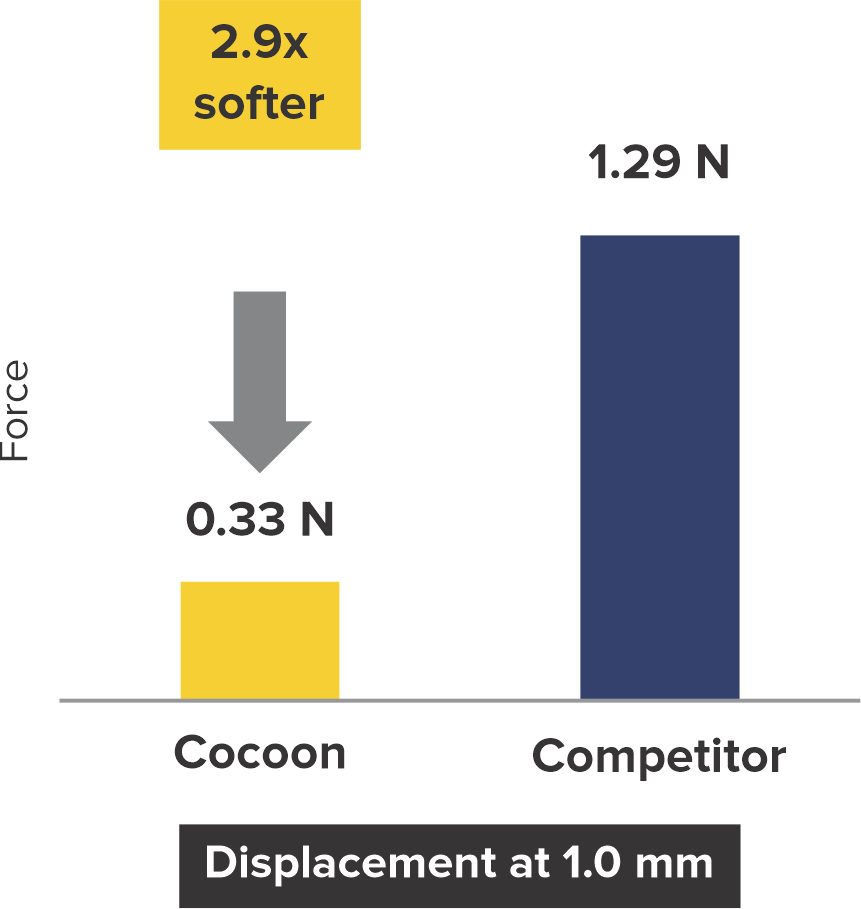
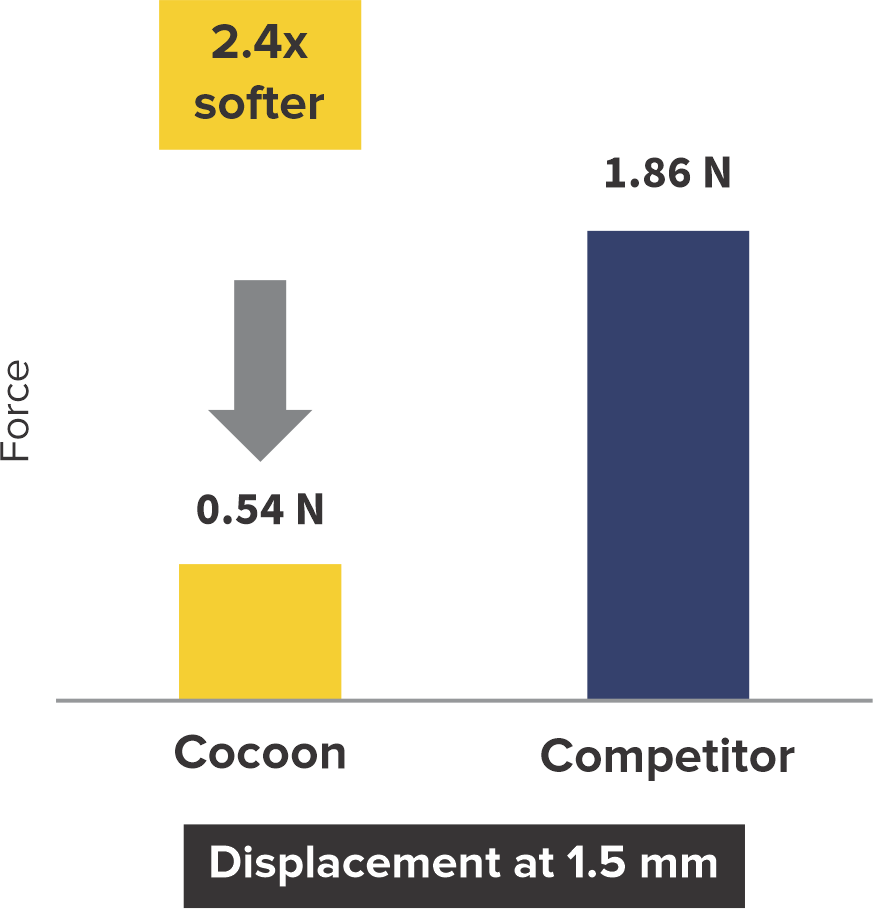
Softness to Protect*
2+ times softer at the rims
5 out of 6 occluders are 8F sheath compatible
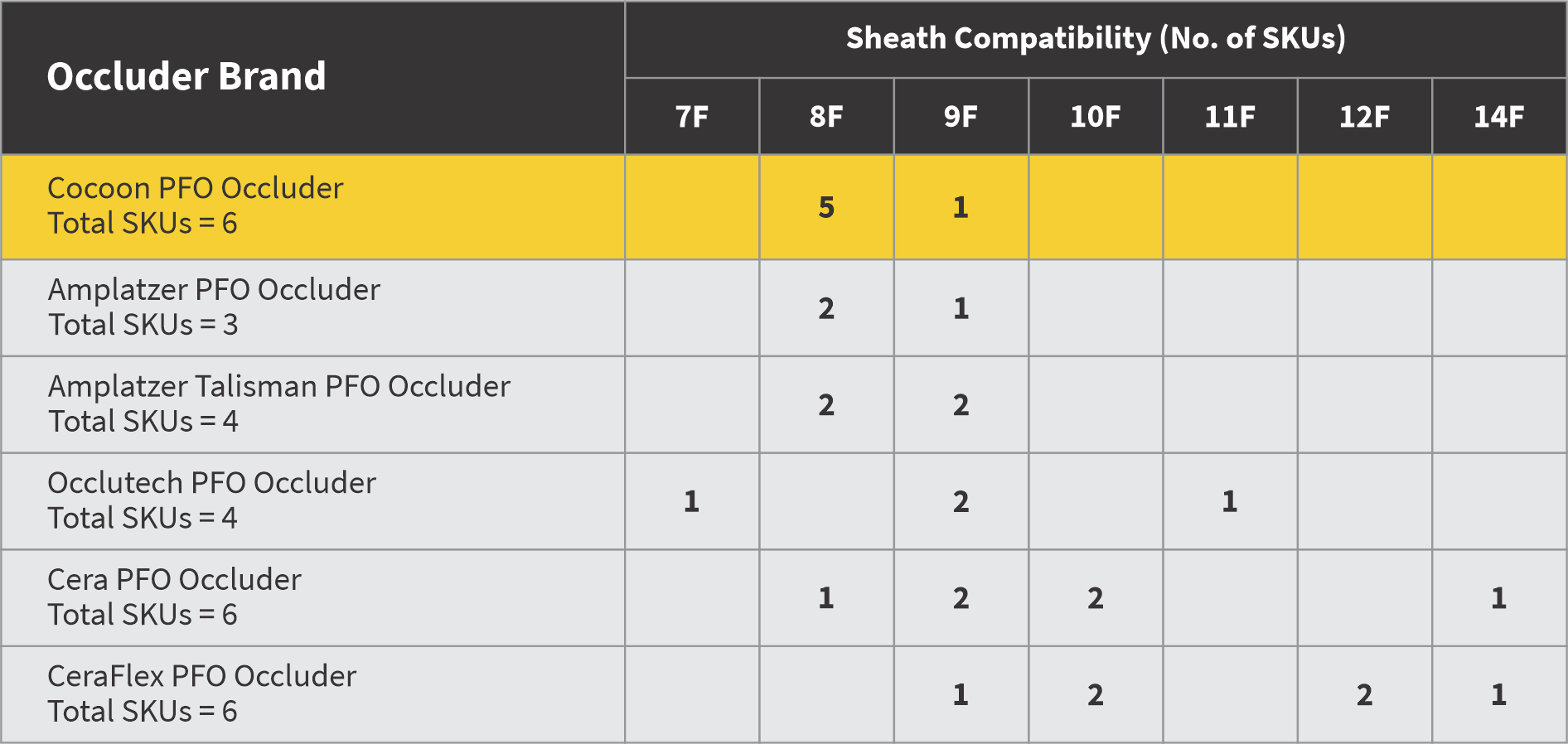
5 out of 6 occluders are 8F sheath compatible
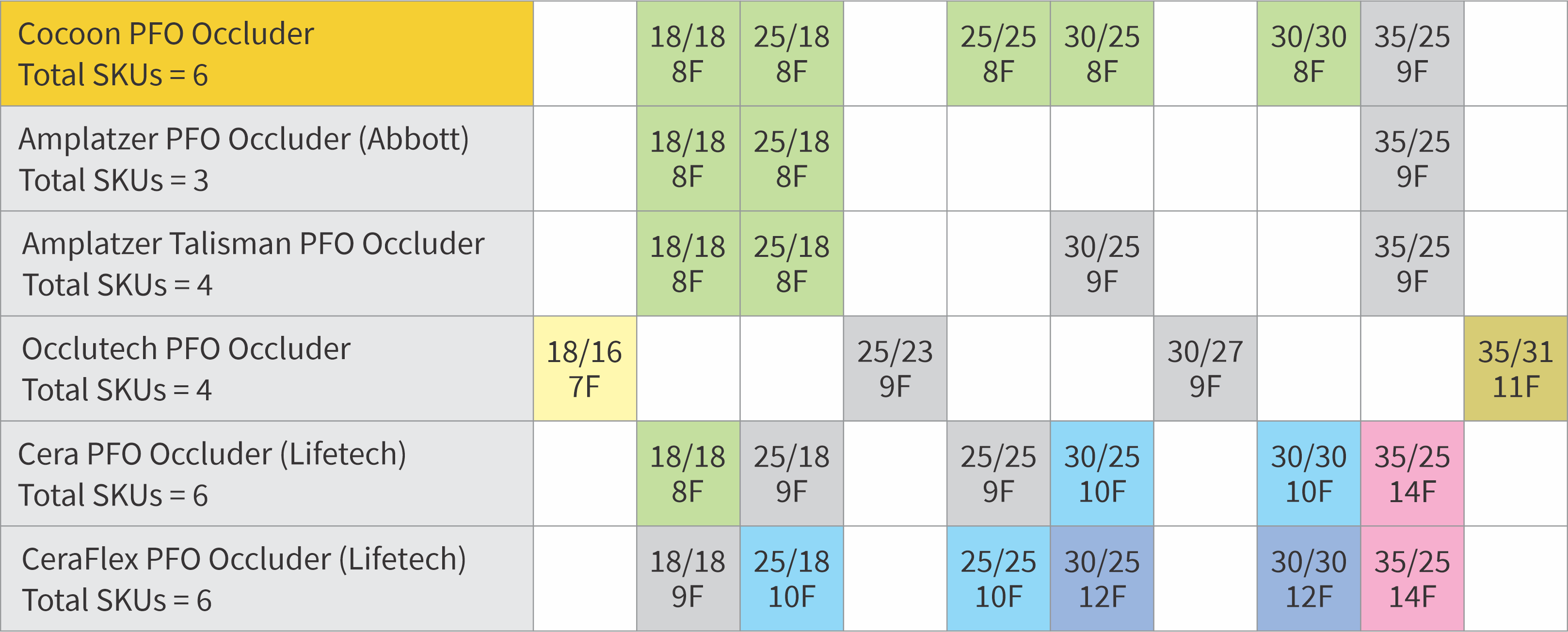
Designed to minimize nickel leaching
Platinum nanocoating
Platinum nanocoating
- Ultrathin layer of Platinum atoms using nanofusion technology is coated on the Nitinol wire by a process called plasma deposition
- Platinum nanocoating makes the Cocoon Occluders inert, biocompatible, non-corrosive and non-allergic and also enhances radiopacity
Platinum nanocoating makes the Cocoon PFO Occluder:
Biocompatible
Non-corrosive
Non-allergic
More radiopaque
Device Description
- Self-expanding, self-centering double disc device with unique Platinum nanocoating
- Device is filled with polypropylene fabric to assist thrombogenicity
- Ability to recapture and redeploy*
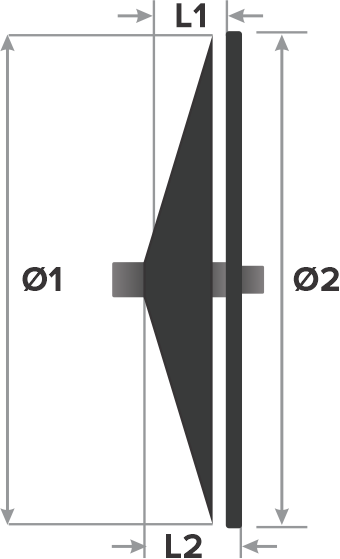
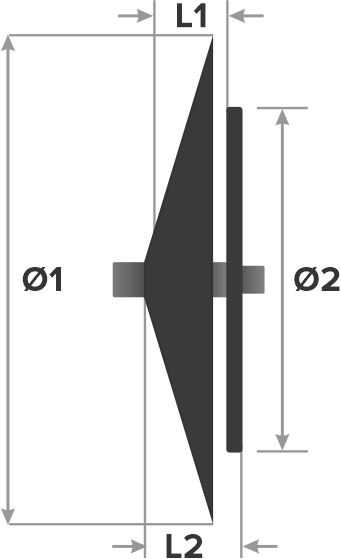
Design:
- Right atrial disc diameter (Ø1)
- Left atrial disc diameter (Ø2)
- Waist length (L1)
- Total length (L2)
*Recapture and redeployment is possible only if delivery cable is securely connected to the disc.
Technical specifications
| Catalog number | Right atrial disc diameter (Ø1) (mm) |
Left atrial disc diameter (Ø2) (mm) |
Waist length (L1) (mm) |
Total length (L2) (mm) |
Sheath Size (F) | Cable Code |
|---|---|---|---|---|---|---|
| CPF18 | 18 | 18 | 3 | 5 | 8 | 6 |
| CPF25 | 25 | 18 | 3 | 5 | 8 | 6 |
| CPF2525 | 25 | 25 | 3 | 5 | 8 | 6 |
| CPF3025 | 30 | 25 | 3 | 5 | 8 | 6 |
| CPF30 | 30 | 30 | 3 | 5 | 8 | 6 |
| CPF35 | 35 | 25 | 3 | 5 | 9 | 6 |
Sizing recommendations
| Distance from defect to aortic root or SVC** (Consider shortest distance) | Suggested Cocoon PFO*** device size |
|---|---|
| Less than 9 mm | Do not implant |
| Between 9 and 12.4 mm | CPF18 |
| Between 12.5 and 14.9 mm | CPF25 |
| CPF2525 | |
| Between 15 and 17.4 mm | CPF3025 |
| CPF30 | |
| More or equal to 17.5 mm | CPF35 |
Consider shortest distance from defect to aortic root or distance from defect to superior vena cava orifice (mm)
Usable length (cm)
| 8F | 9F | |
|---|---|---|
| Sheath | 78 | 78 |
| Dilator | 83 | 83 |
| Loader | 10 | 12 |
Delivery cable
| Description | Profile (inch) | Length (cm) | Catalog Number |
|---|---|---|---|
| 6F Delivery Cable | 0.077 | 117 | CDC6F |
Preclinical studies
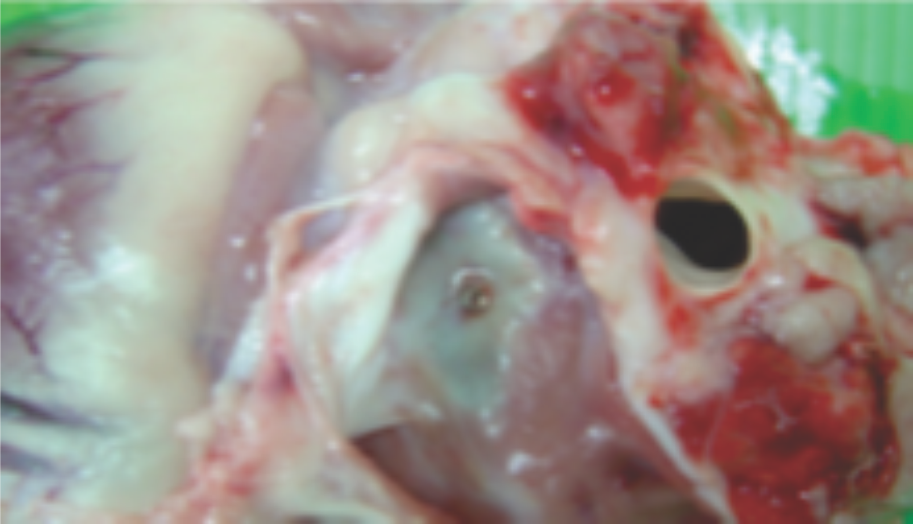
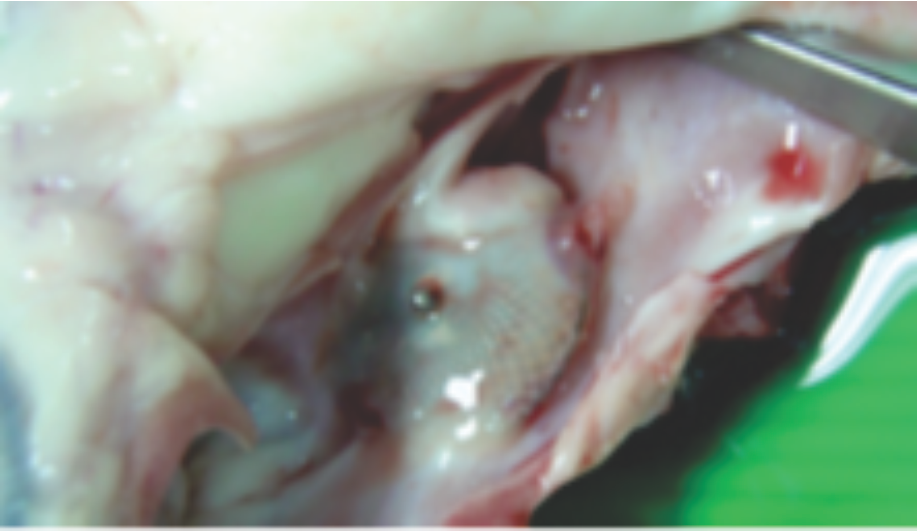
Images from pre-clinical trials of closure device depicting neoendothelialization.Pigs were sacrificed at 36 and 42 days.
*Data on SMT file. R & D tests performed using 18/18 mm PFO Occluders for both devices. The test results are an average of the test performed three times.
**SVC : Superior Vena Cava
**PFO : Patent Foramen Ovale
##TTE : Trans Thoracic Echocardiogram
#Recapture and redeployment is possible only if delivery cable is securely connected to the disc
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the "Instructions for use" supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the “Instructions for use” supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precaution. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA and Japan.
Check the regulatory status of the device before distribution in areas where CE marking is not the regulation in force. Tests performed by and data on file at Sahajanand Medical Technologies Limited (SMT). Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Sahajanand Medical Technologies Limited.
Cocoon PFO Occluder is manufactured by Vascular Innovations Co., Ltd. Thailand, a SMT group company. Cocoon is a trademark of Vascular Innovations Co., Ltd. Amplatzer PFO Occluder & Amplatzer Talisman PFO Occluder are trademarks of Abbott Group of companies. Occlutech PFO Occluder is a trademark of Occlutech International AB. Cera PFO Occluder & Ceraflex PFO Occluder are trademarks of Lifetech Scientific.
Cocoon range of occluders are currently not approved by USFDA and are not available for sale in USA.
Competitor information collected on 20th June 2023 from following sources
Abbott PFO Occluder & Abbott Amplatzer Talisman PFO Occluder:https://manuals.sjm.com/Search-Form?re=North-America&cc=US&ln=EN&ct=professional&fam=861f73cb-e36b-4f06-a58f-4b11fa263dcf&cat=71260c89-7cb8-475d-950b-0262191e7526&seg=dd28d64f-7d0b-4660-aa2c-da987bb7894c&qry=Amplatzer%20PFO%20occluder&ipp=10
Occlutech PFO Occluder: https://occlutech.com/files/brochure/Occlutech_PFO_Br.pdf
Cera PFO Occluder: http://www.lifetechmed.com/en/product/p1/cera/385.aspx
CeraFlex PFO Occluder:
http://www.lifetechmed.com/en/product/p1/ceraflex%E2%84%A2%20occluders/360.aspx
Disclaimer: © 2025 Sahajanand Medical Technologies Limited – All rights reserved. Specifications are subject to modification, revision and improvement.


