-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
Maximum Circular Unsupported Surface Area (MCUSA)
- MCUSA = 0.73 mm2 (Value measured on expanded stent of 2.5 mm)
- Lower MCUSA aids in uniform drug distribution
- Scaffolding is more uniform
Scaffolding Overview and Design Characteristics
- Provides enhanced structural support
- Aids in optimal apposition to vessel wall
- Prevents plaque prolapse
- Leads to uniform expansion of stent
Long Link
- Enhanced overall structural integrity
- Longer links aid in well controlled expansion of the stent
- Longer link resists longitudinal compression
- Longer link transmits "Push" force with higher efficiency
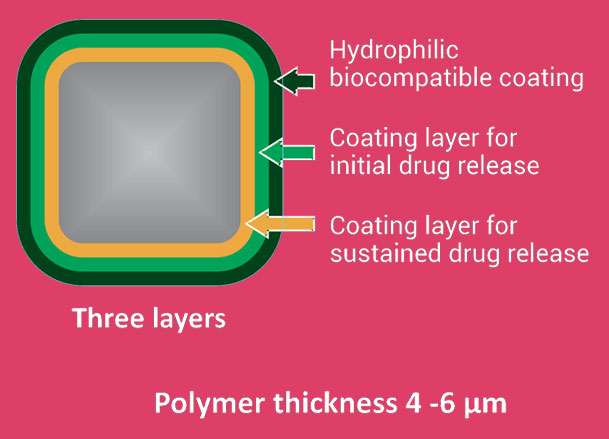
Unique biodegradable polymer matrix
- Combination of hydrophilic and hydrophobic polymers in form of thin coating layers on stent
- Provides control over drug release
- Ensures excellent coating integrity
Uses clinically proven, safe and effective drug Sirolimus elutes from biodegradable polymeric matrix
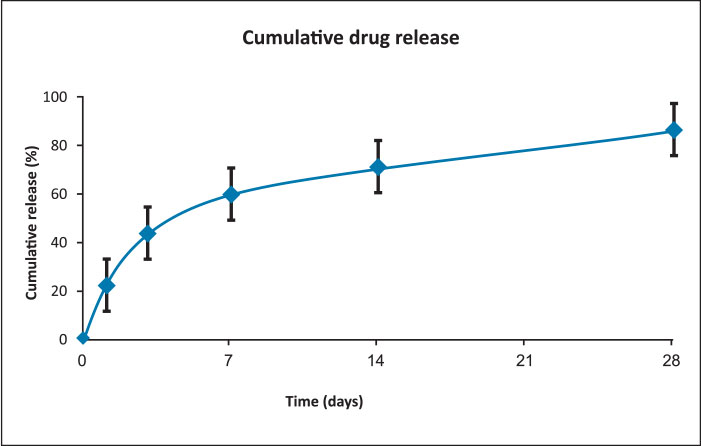
Drug release profile
- Drug: Sirolimus
- Drug dose: 1.4 µg/mm2
- Release kinetics
- About 80% of drug is released at 4 weeks in biological media while 100% drug is released at a slow rate within 3 months.
- The initial moderate level of Sirolimus drug release from middle layer coating helps to inhibit early phase of neointimal hyperplasia.
- Controlled drug release kinetics from base layer coating is beneficial to maintain the effective amount of drug level in the arterial tissues which are required to prevent smooth muscle cell proliferation.
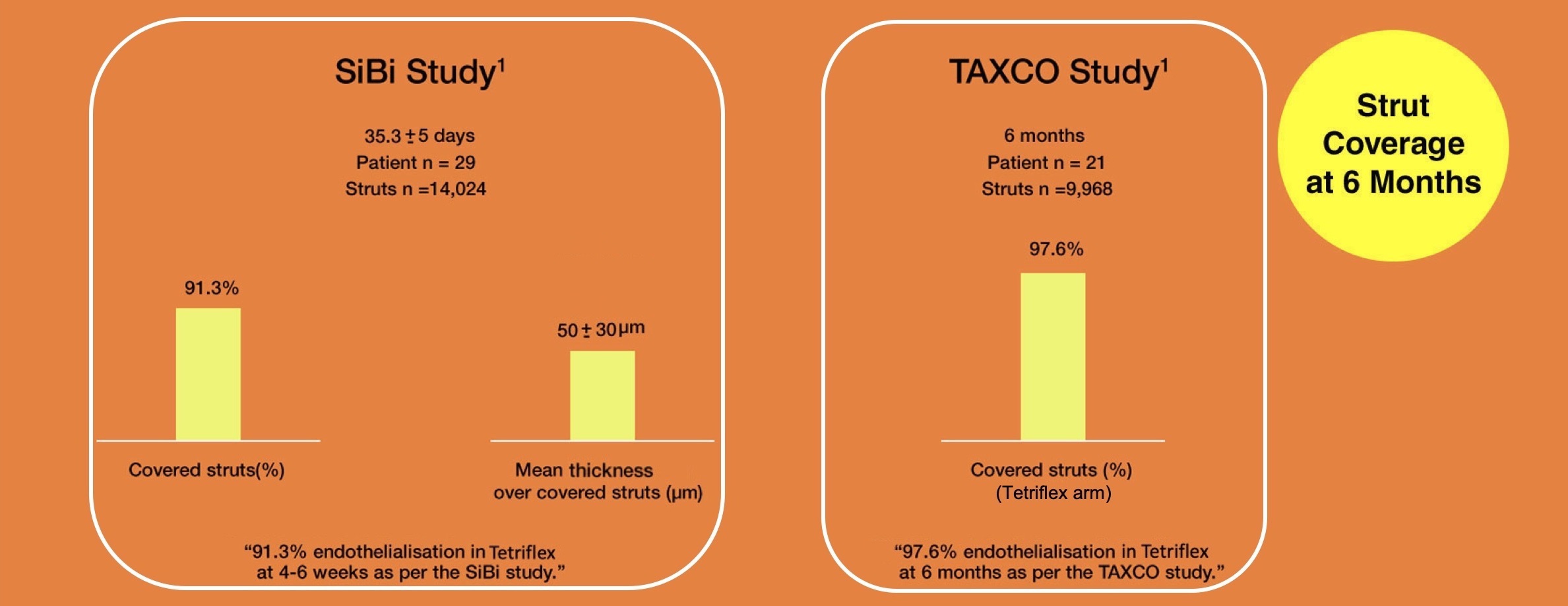
Clinical evidence from OCT study
NIH: Neointimal hyperplasia
1.Abhyankar, A, Abizaid, A, Chamié, D, Patel, G. SiBi optical coherence tomography study. Catheter Cardiovasc Interv. 2020; 1– 8. https://doi.org/10.1002/ccd.29371
2. Presented at EuroPCR 2019, 22 May 2019 12:15 - 13:15 Room 243 / Level 2
Tetriflex in RCA - PDA tortuous vessel

RCA severely tortuous vessel and lesion PDA 80% stenosis
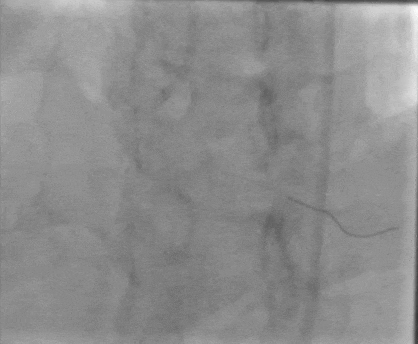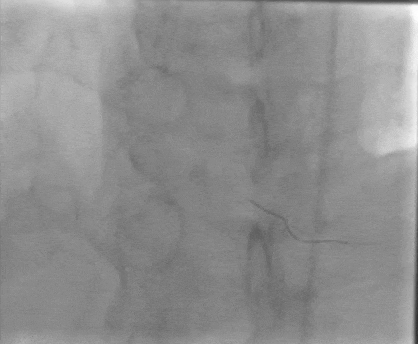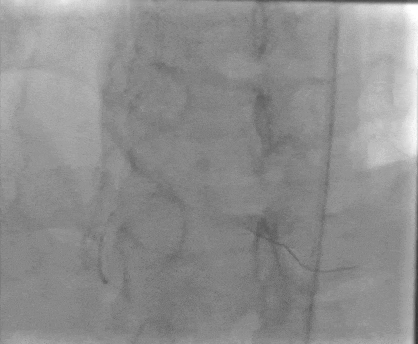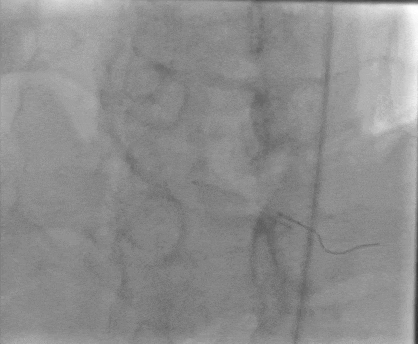
Tetriflex 2.50mm x 24mm negotiated in RCA-PDA
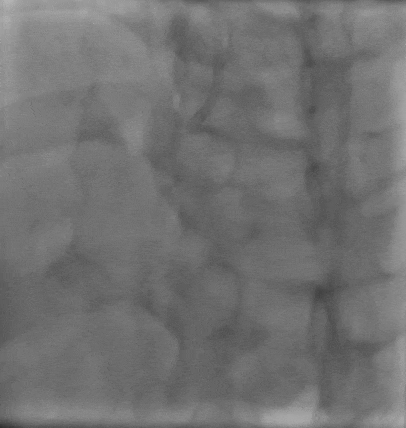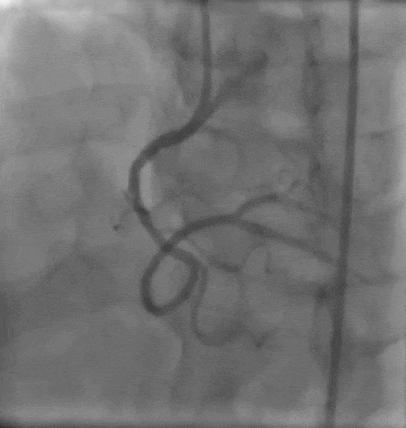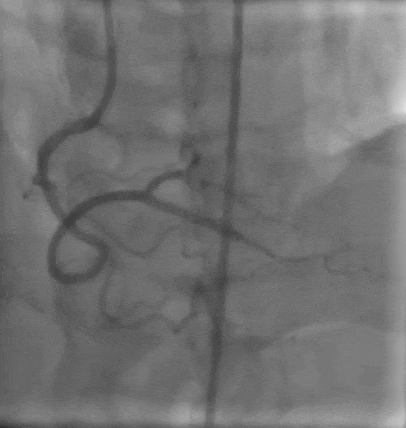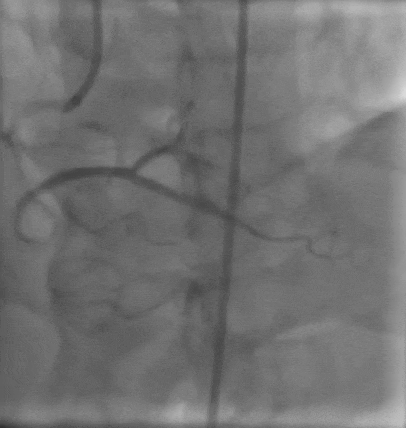
Post stent TIMI
Tetriflex is a trademark of Sahajanand Medical Technologies Pvt. Ltd. or its affiliates. Specifications are subject to modification, revision and improvement.
BioFreedom and BioMatrix Alpha are trademarks of Biosensors International. Xience V, Xience Alpine, Xience Prime, Xience Xpedition and Xience Sierra are trademarks of the Abbott Group of Companies. Resolute Onyx is a trademark of Medtronic, Inc. or it's affiliates. Synergy is a trademark of Boston Scientific Corporation or its affiliates. Ultimaster is a trademark of Terumo Corporation. Orsiro is a trademark of Biotronik SE.



