-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
Uniform distribution of coating on balloon surface
SEM images of the coating on the surface
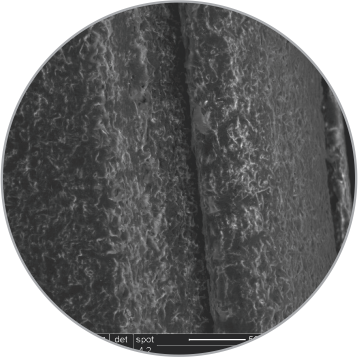
Low magnification
(200x)
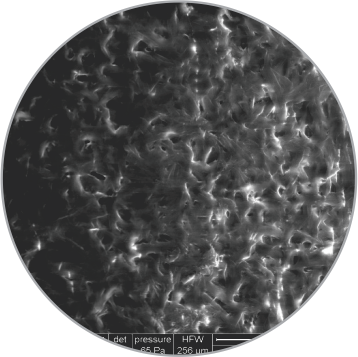
High magnification
(1000x)
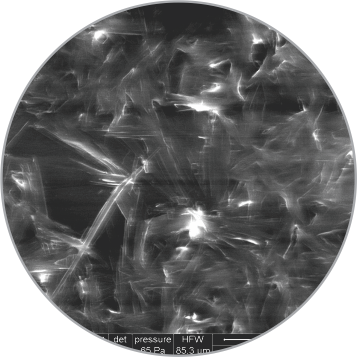
High magnification
(3000x)
Uniform coating surface of Peripherics
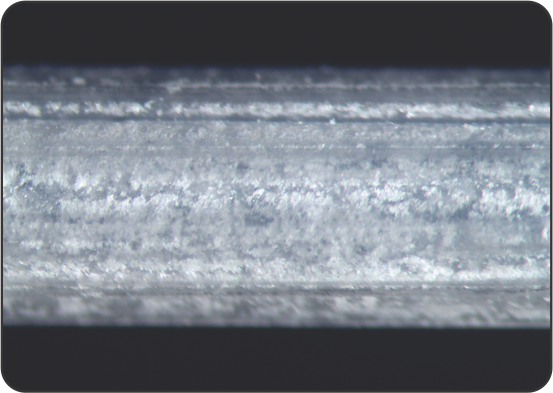

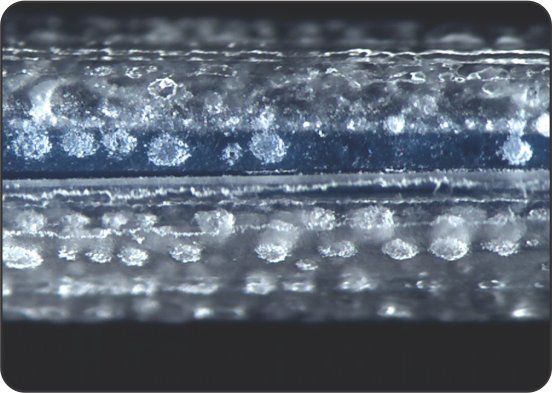
Competitor 1
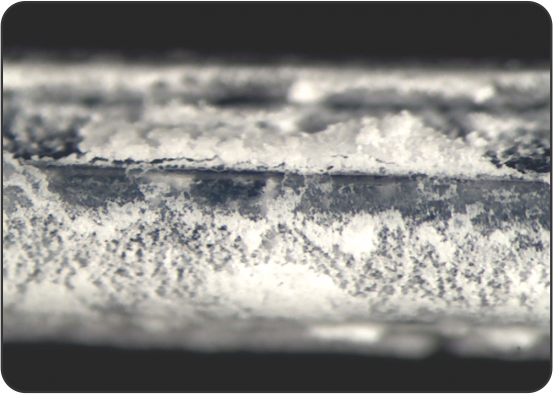
Competitor 2

Competitor 3
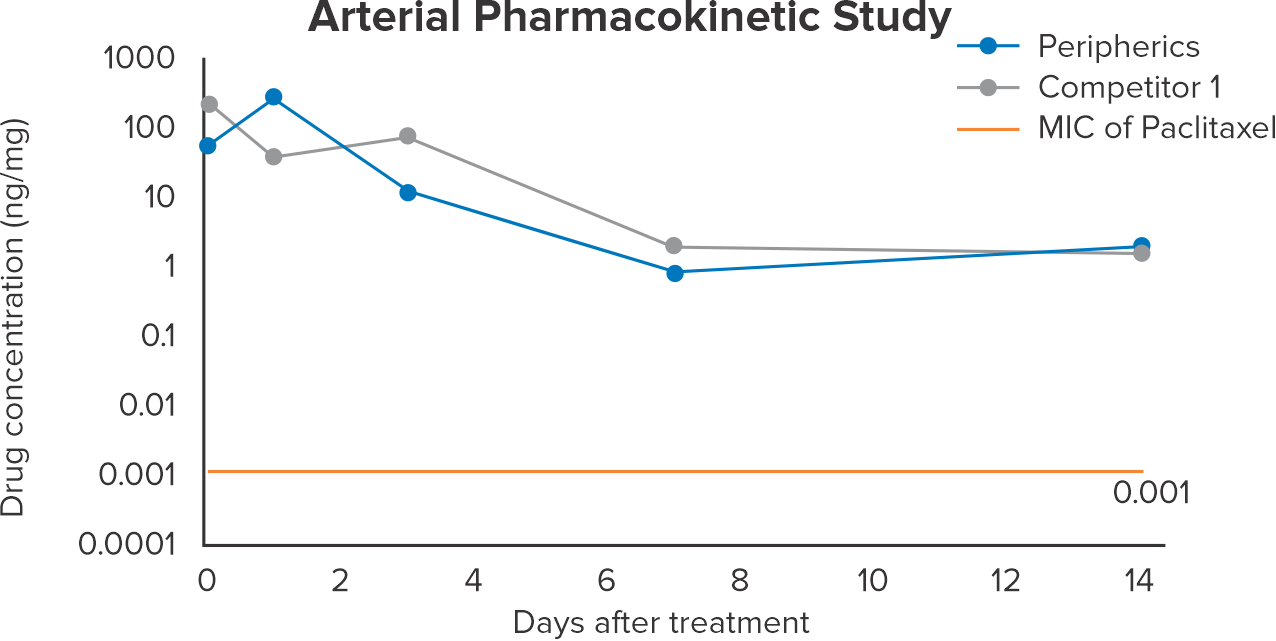
Paclitaxel* concentration in arterial tissue up to 14 days
Semi-crystalline paclitaxel
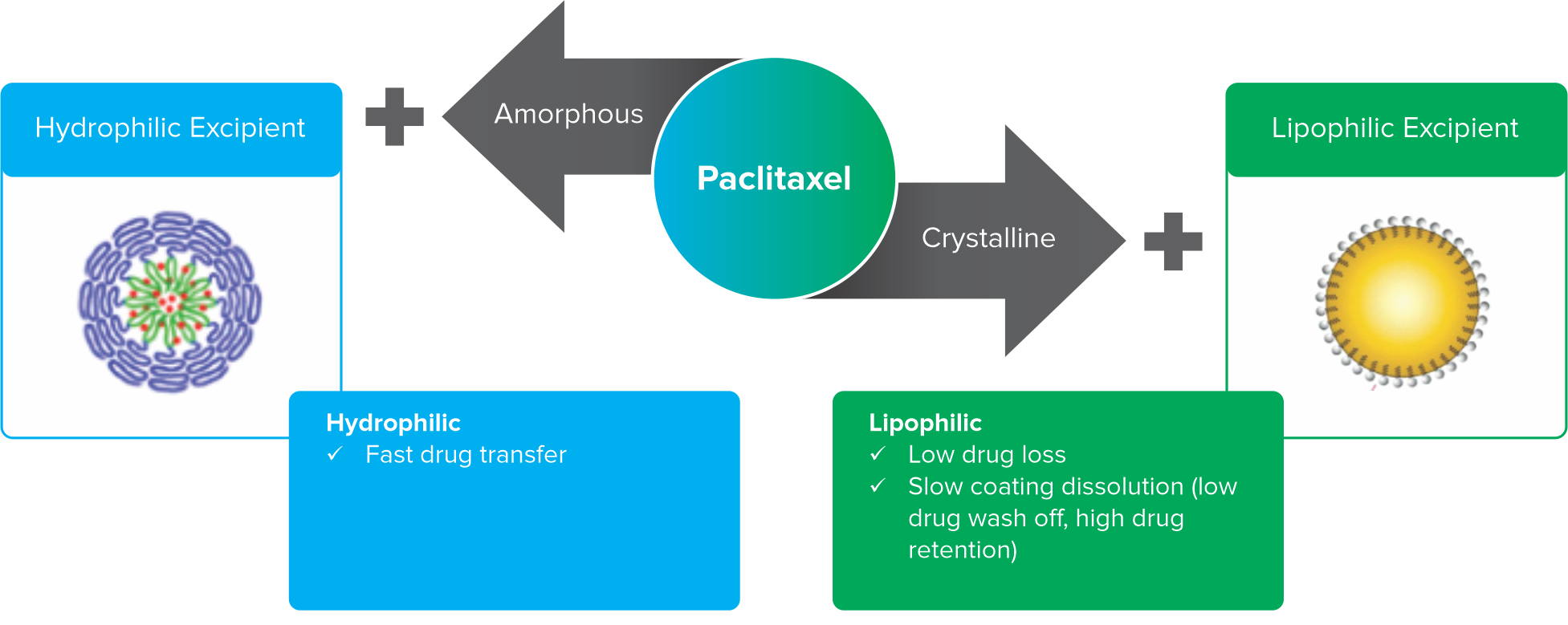
Coating process
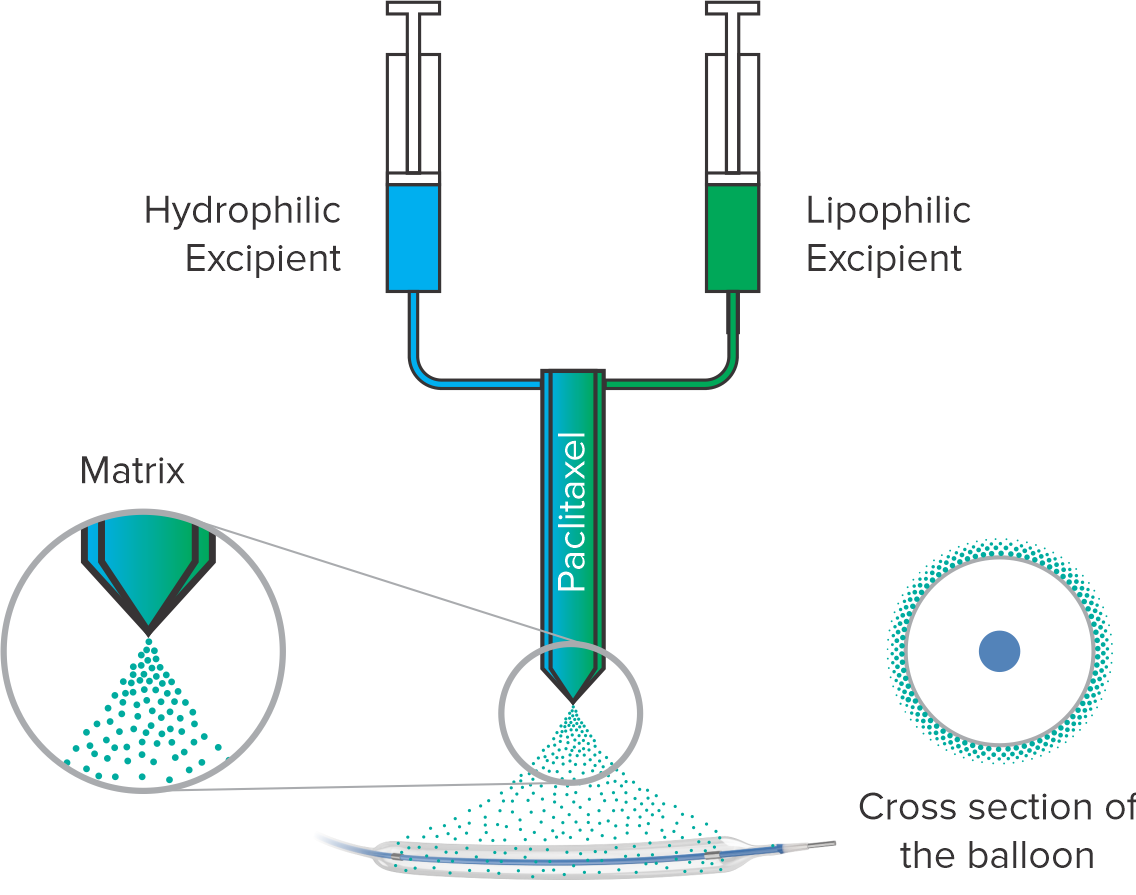
Coating on balloon
Paclitaxel drug load of 2 μg/mm2
Proprietary coating technology

- Proprietary balloon design
- Balloon material has been conceptualized to maximise force transmission
- Balloon has been designed to maximise the drug transfer during inflation
- Optimal adhesion of coating to balloon with minimal loss during transit to lesion
- Desired wall apposition to enable effective and uniform drug delivery
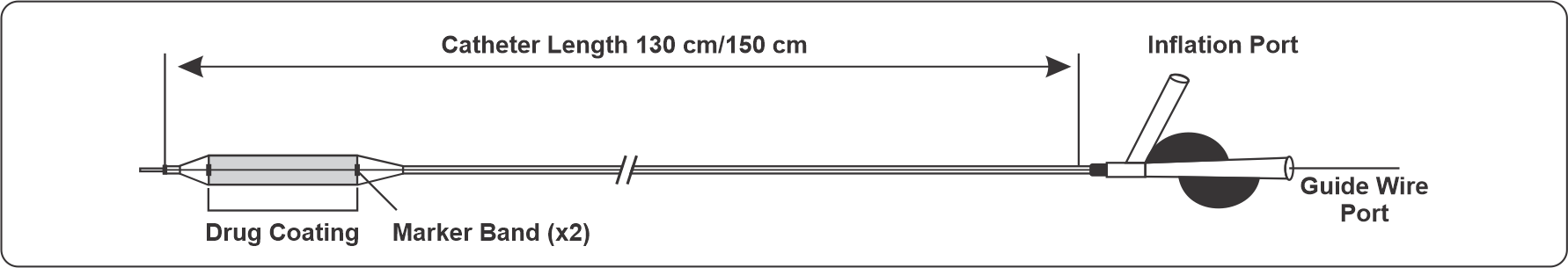
Technical specifications
| Catheter type | OTW (BTK) | OTW (SFA) |
|---|---|---|
| Recommended guide wire | 0.018" | 0.035" |
| Tip | Soft and tapered | Soft and tapered |
| Balloon markers | Two radiopaque markers | Two radiopaque markers |
| Usable length | 150 cm | 130 cm |
| Recommended introducer sheath | 2-4 mm: 4 F | 5-6 mm: 6 F & 7 mm: 7 F |
| Nominal pressure (NP) | 10 atm | 7 atm |
| Rated burst pressure (RBP) | 14 atm | 11 atm |
Size matrix: 0.018” OTW (BTK)
| Balloon Diameter (mm) |
Balloon Length (mm) | |||
|---|---|---|---|---|
| 80 | 100 | 120 | 150 | |
| 2.00 | ||||
| 2.50 | ||||
| 3.00 | ||||
| 4.00 | ||||
Size matrix: 0.035” OTW (SFA)
| Balloon Diameter (mm) |
Balloon Length (mm) | ||
|---|---|---|---|
| 100 | 120 | 150 | |
| 5.00 | |||
| 6.00 | |||
| 7.00 | |||
Colored Sizes are make to order
BTK- Below the Knee, OTW - Over the Wire, SFA - Superficial Femoral Artery, SEM- Scanning Electron Microscope, MIC - Minimum Inhibitory Concentration,
PTA - Percutaneous Transluminal Angioplasty
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the "Instructions for use" supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As a part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.
*Tests performed by and data on file at Sahajanand Medical Technologies Limited (SMT). Illustrations are artist's representations only and should not be considered as engineering drawings or photographs. Photos on file at Sahajanand Medical Technologies Limited.
Peripherics is a trademark of Sahajanand Medical Technologies Limited (SMT). Peripherics is currently not approved by USFDA and is not available for sale in USA.
Disclaimer: © 2022 Sahajanand Medical Technologies Limited - All rights reserved. Specifications are subject to modification, revision and improvement.


