-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
Platinum nanocoating technology
Nitinol wire used in the Cocoon VSD Occluder is coated with Platinum atoms using nano fusion technology by plasma deposition
Raw nitinol
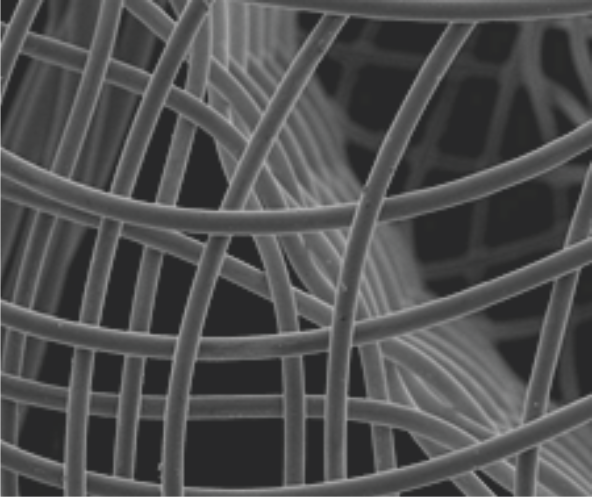
Electropolished
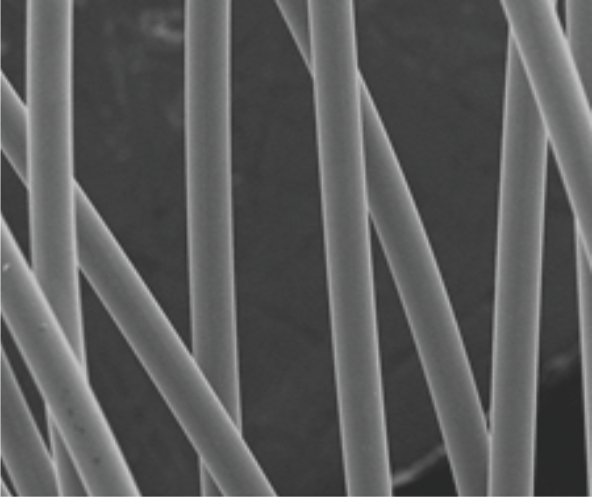
Platinum nanocoating
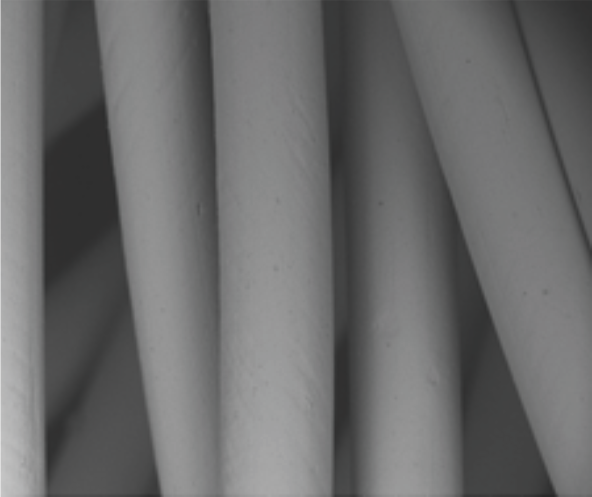
Platinum nanocoating makes the Cocoon VSD Occluder:
Biocompatible
Non-corrosive
Non-allergic
More radiopaque
Key highlights
- Self-expanding, double disc device which with unique Platinum nanocoating
- 4, 7, & 10 mm waist lengths available
- Available in multiple lengths to match the types and locations of the ventricular septal defects to close membranous and muscular defects
- Discs are filled with polypropylene fabric which assists thrombogenicity
- Ability to recapture and redeploy*
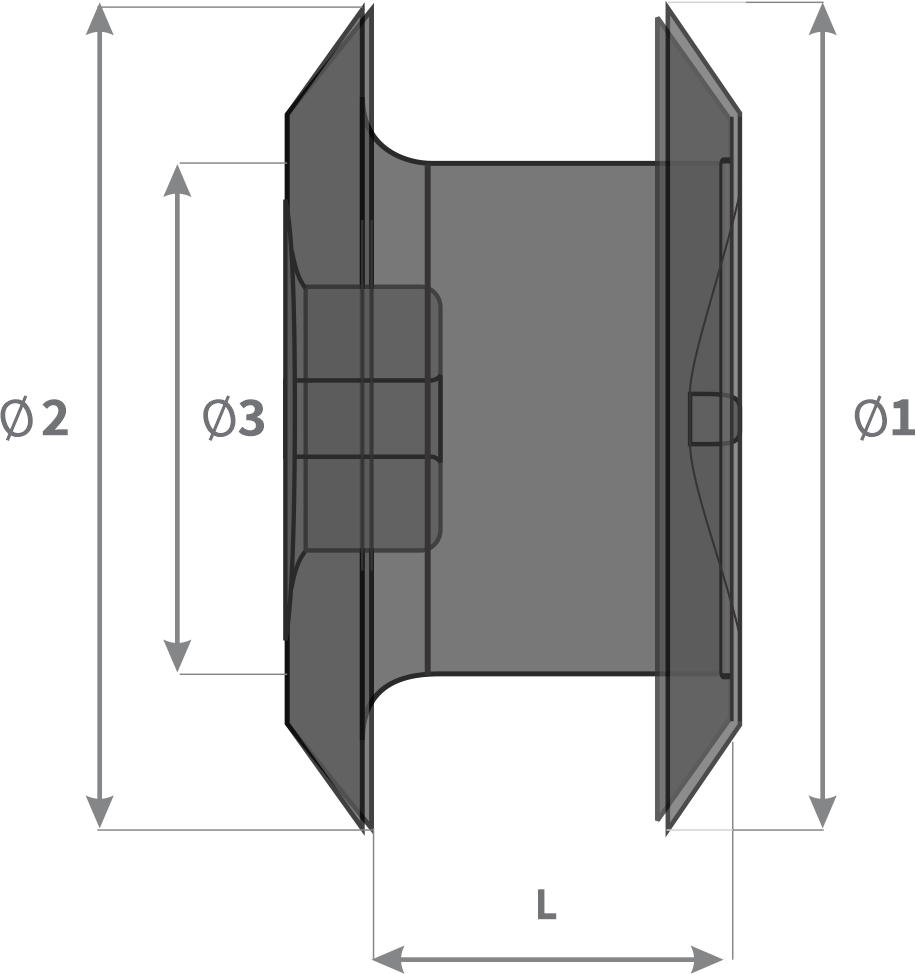
Design:
- Left ventricular disc diameter (Ø1)
- Right ventricular disc diameter (Ø2)
- Waist diameter (Ø3)
- Waist length (L)
Thickness of the septum is considered for selecting the length of the device
*Recapture and redeployment is possible only if delivery cable is securely connected to the disc.
Technical specifications
| Product Code | Left ventricular disc diameter (Ø 1) (mm) |
Right ventricular disc diameter (Ø 2 ) (mm) |
Waist diameter (Ø3) (mm) |
Waist length (L) (mm) |
Recommended for VSD size | Accessory catalog number |
|---|---|---|---|---|---|---|
| COV0404 | 10 | 10 | 3.25 | 4 | 3 mm | COV6F or COVII6F |
| COV0604 | 12 | 12 | 5.25 | 4 | 3.1 to 5 mm | COV6F or COVII6F |
| COV0804 | 14 | 14 | 7.25 | 4 | 5.1 to 7 mm | COV6F or COVII6F |
| COV1004 | 16 | 16 | 9.25 | 4 | 7.1 to 9 mm | COV7F or COVII7F |
| COV1204 | 18 | 18 | 11.25 | 4 | 9.1 to 11 mm | COV7F or COVII7F |
| COV0407 | 10 | 10 | 4 | 7 | 2 mm | COV6F or COVII6F |
| COV0607 | 12 | 12 | 6 | 7 | 2.1 to 4 mm | COV6F or COVII6F |
| COV0807 | 14 | 14 | 8 | 7 | 4.1 to 6 mm | COV6F or COVII6F |
| COV1007 | 16 | 16 | 10 | 7 | 6.1 to 8 mm | COV7F or COVII7F |
| COV1207 | 18 | 18 | 12 | 7 | 8.1 to 10 mm | COV7F or COVII7F |
| COV0410 | 10 | 10 | 4 | 10 | 2 mm | COV6F or COVII6F |
| COV0610 | 12 | 12 | 6 | 10 | 2.1 to 4 mm | COV6F or COVII6F |
| COV0810 | 14 | 14 | 8 | 10 | 4.1 to 6 mm | COV6F or COVII6F |
| COV1010 | 16 | 16 | 10 | 10 | 6.1 to 8 mm | COV7F or COVII7F |
| COV1210 | 18 | 18 | 12 | 10 | 8.1 to 10 mm | COV7F or COVII7F |
Usable lengths of compatible accessories (cm)
| Usable Length (cm) | ||
|---|---|---|
| 6F | 7F | |
| Sheath | 83 | 83 |
| Dilator | 89 | 89 |
| Loader | 9 | 9 |
Delivery cable & sheath
| Catalog number | Description | Diameter (inch) | Length (cm) | |
|---|---|---|---|---|
| CDCV | ||||
| Delivery cable | 0.030 | 140 | ||
| Delivery cable sheath | ID | OD | 125 | |
| 0.055 | 0.079 | |||
Delivery cable: View 1

Delivery cable: View 2

Delivery cable sheath

Preclinical studies*
Ventricular Septal Defect was created in swine models and subsequently implanted with a Cocoon VSD Occluder
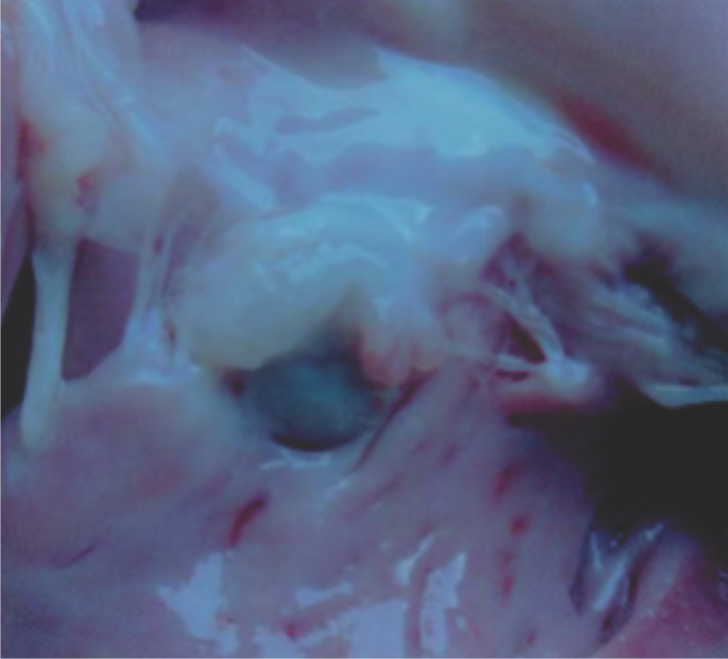
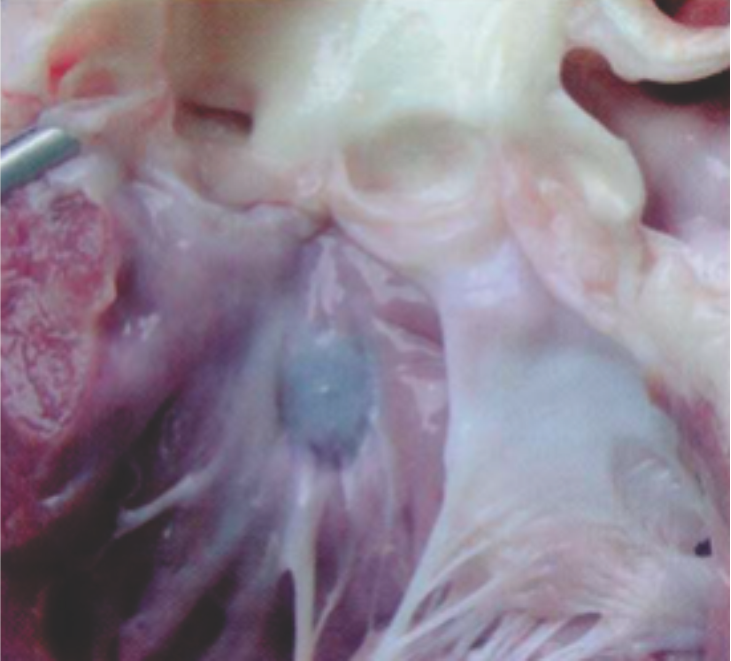
Explants at 30 days show the device incorporated with tissues
Preclinical studies*
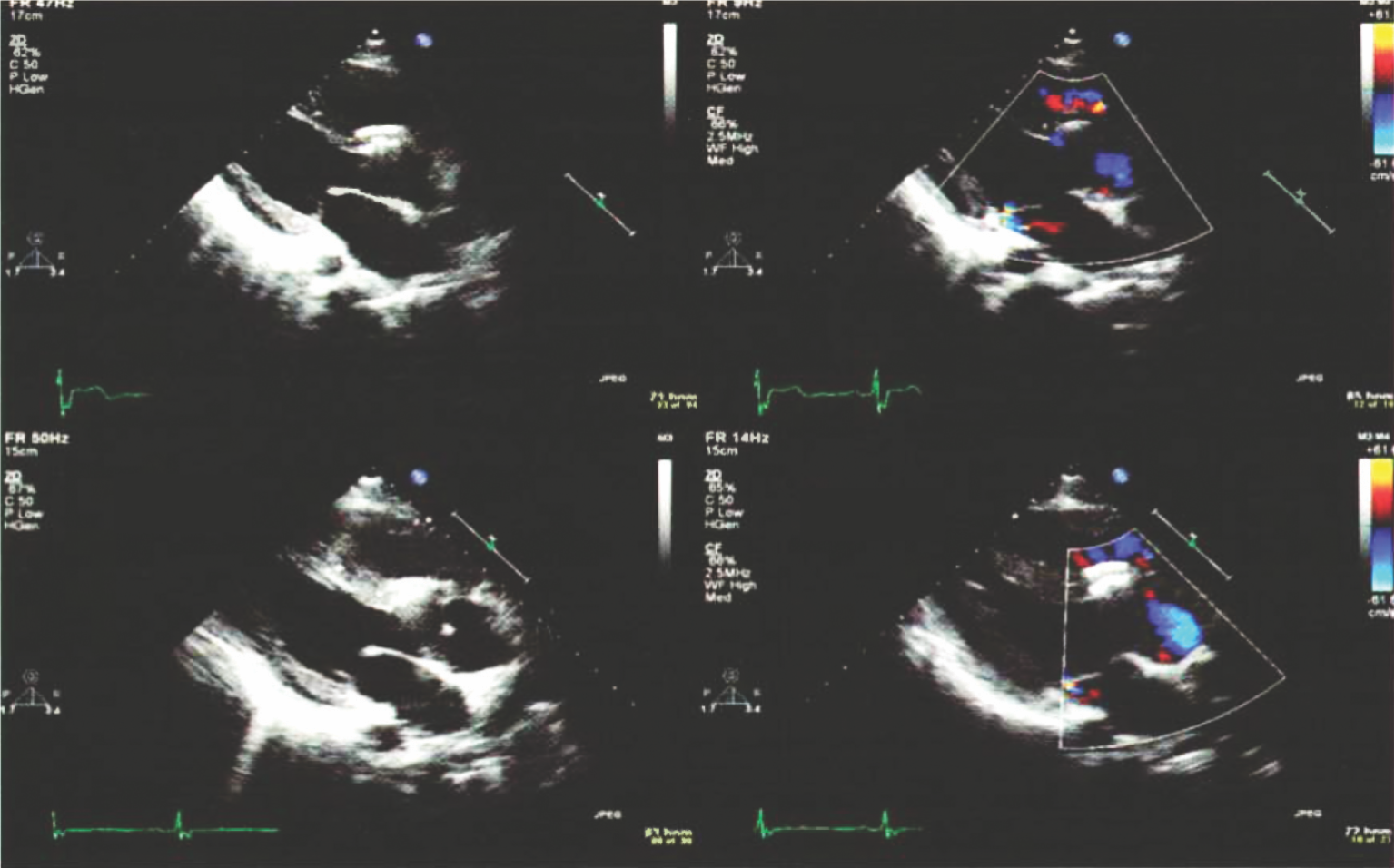
Echo images of the swine at a day 1 and day 30 showing the complete closure of the defect
VSD : Ventricular Septal Defect
*Data on SMT file.
#Recapture and redeployment is possible only if delivery cable is securely connected to the disc
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the "Instructions for use" supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.
Check the regulatory status of the device before distribution in areas where CE marking is not the regulation in force. Tests performed by and data on file at Sahajanand Medical Technologies Limited (SMT). Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Sahajanand Medical Technologies Limited.
Cocoon VSD Occluder is manufactured by Vascular Innovations Co., Ltd. Thailand, a SMT group company. Cocoon is a trademark of Vascular Innovations Co., Ltd.
Cocoon range of occluders are currently not approved by USFDA and are not available for sale in USA.
Disclaimer: © 2025 Sahajanand Medical Technologies Limited – All rights reserved. Specifications are subject to modification, revision and improvement.


