-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- Investors
- About SMT
Main navigation
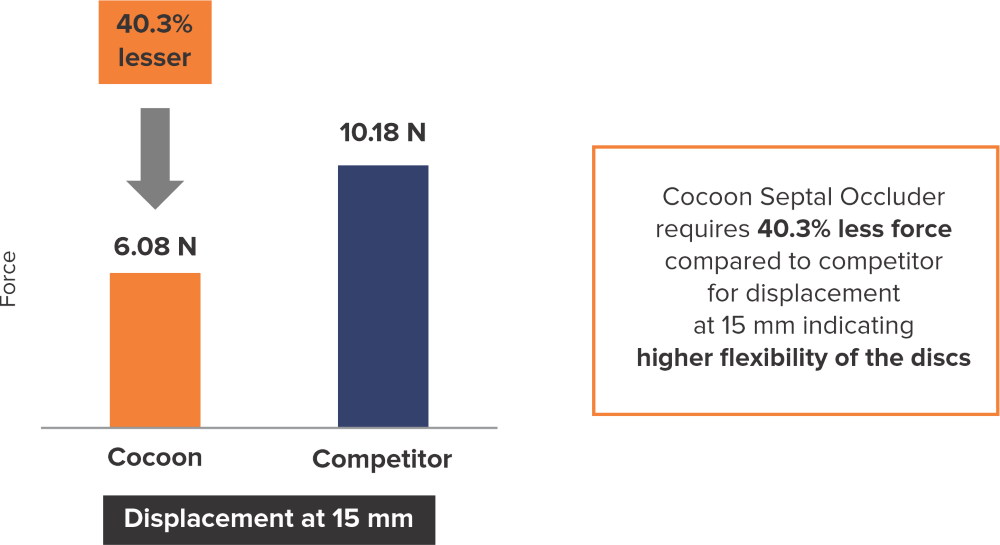
Flexibility at the disc level*
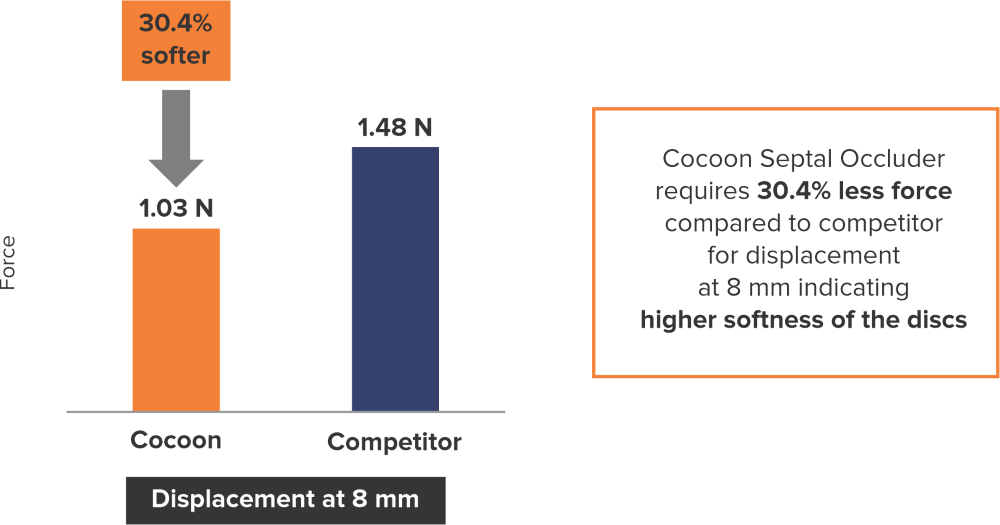
Softness to Protect*
Conformability at the discs*
Right disc
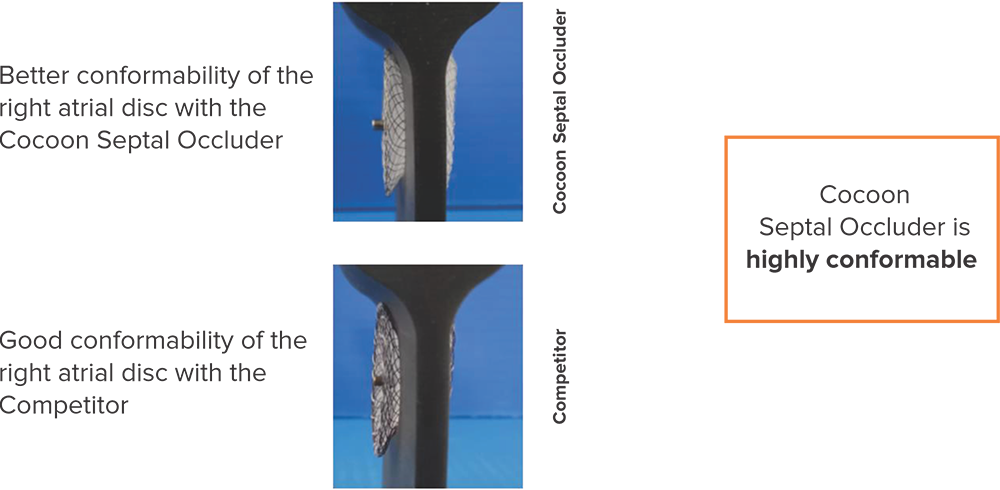
Left disc

Designed to minimize nickel leaching
Platinum nanocoating
Platinum nanocoating
- Ultrathin layer of Platinum atoms using nanofusion technology is coated on the Nitinol wire by a process called plasma deposition
- Platinum nanocoating makes the Cocoon Occluders inert, biocompatible, non-corrosive and non-allergic and also enhances radiopacity
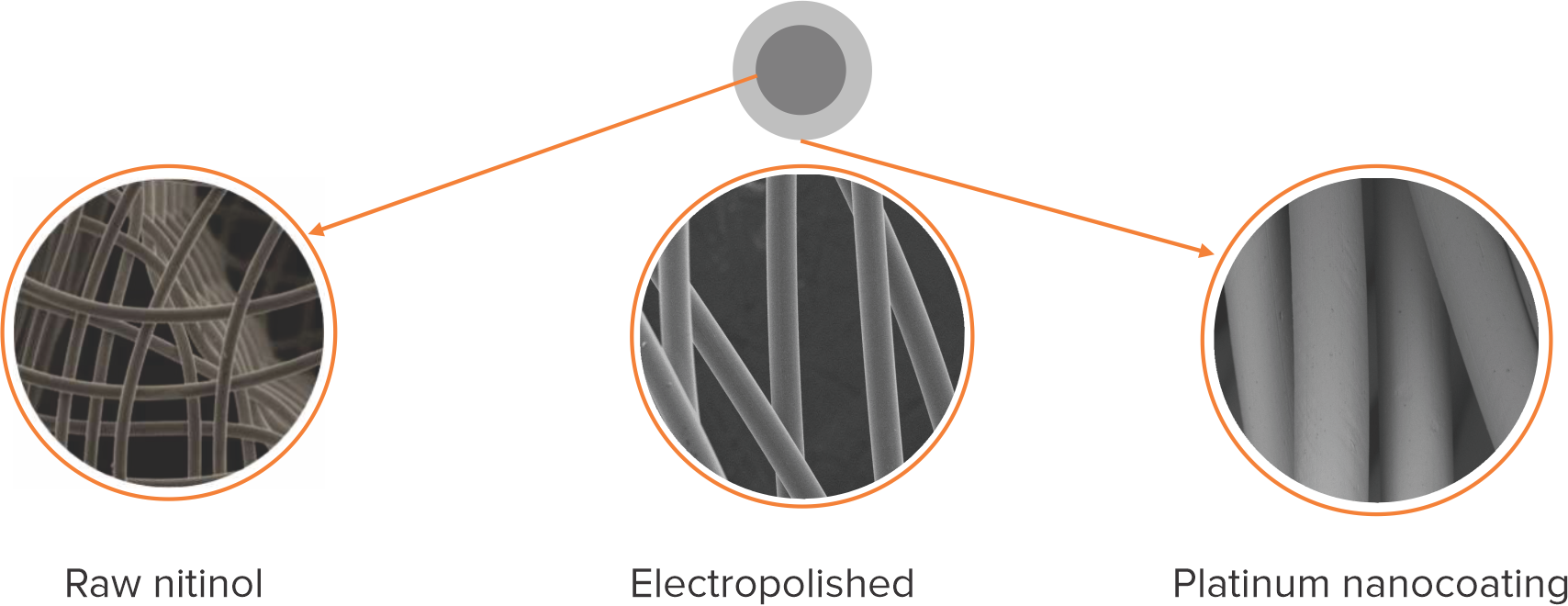
Platinum nanocoating makes the Cocoon Septal Occluder:
Biocompatible
Non-corrosive
Non-allergic
More radiopaque
Optimal Sealing of Defects

The waist of Cocoon Septal Occluder has adequate sealing strength at the level of the discs: Up to 100% immediate closure rates1, 2
Device Description
- Self-expanding, self-centering, double disc device with unique Platinum nanocoating
- Discs are filled with polypropylene fabric which assists thrombogenicity
- Ability to recapture and redeploy*
- 0% erosion up to 43 months of follow-up in more than 4000 patients3
- 95% to 100% device success rate from early European experience2,4
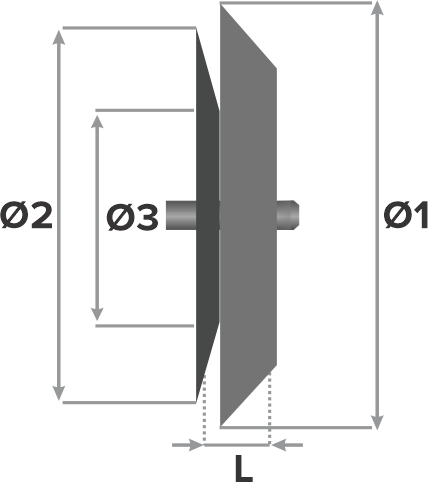
Design:
- Left atrial disc diameter (Ø1)
- Device size (Ø3)
- Right atrial disc diameter (Ø2)
- Waist length (L)
Technical specifications
| Product Code | Device size (Ø3) (mm) |
Left atrial disc diameter (Ø1) (mm) |
Waist length (L) (mm) |
Right atrial disc diameter (Ø2) (mm) |
Sheath (F) |
|---|---|---|---|---|---|
| COA08 | 8 | 20 | 3 | 18 | 6-7 |
| COA10 | 10 | 22 | 3 | 20 | 6-7 |
| COA12 | 12 | 26 | 4 | 22 | 8-9 |
| COA14 | 14 | 28 | 4 | 24 | 8-9 |
| COA16 | 16 | 30 | 4 | 26 | 8-9 |
| COA18 | 18 | 32 | 4 | 28 | 10-12 |
| COA20 | 20 | 34 | 4 | 30 | 10-12 |
| COA22 | 22 | 36 | 4 | 32 | 10-12 |
| COA24 | 24 | 38 | 4 | 34 | 10-12 |
| COA26 | 26 | 40 | 4 | 36 | 10-12 |
| COA28 | 28 | 42 | 4 | 38 | 10-12 |
| COA30 | 30 | 44 | 4 | 40 | 12-14 |
| COA32 | 32 | 46 | 4 | 42 | 12-14 |
| COA34 | 34 | 50 | 4 | 44 | 12-14 |
| COA36 | 36 | 52 | 4 | 46 | 12-14 |
| COA38 | 38 | 54 | 4 | 48 | 12-14 |
| COA40 | 40 | 56 | 4 | 50 | 12-14 |
| COA42 | 42 | 58 | 4 | 52 | 14 |
Not available in CE mark countries
Usable lengths of compatible accessories (cm)
| Usable Length (cm) | |||||||
|---|---|---|---|---|---|---|---|
| 6F | 7F | 8F | 9F | 10F | 12F | 14F | |
| Sheath | 60 | 60 | 78 | 78 | 78 | 78 | 78 |
| Dilator | 66 | 66 | 84 | 84 | 84 | 84 | 84 |
| Loader | 9 | 9 | 9 | 11 | 11 | 13 | 13 |
Delivery cable
| Description | Profile (inch) | Length (cm) | Catalog Number |
|---|---|---|---|
| 6F Delivery Cable | 0.077 | 117 | CDC6F |
Preclinical studies
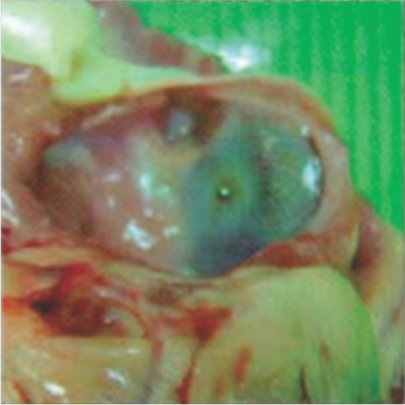
Neoendothelialization on the left atrial surface of the device at 36 days after implantation
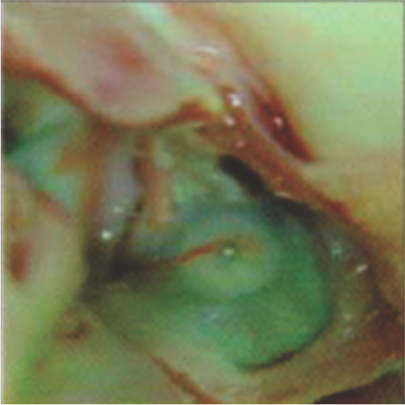
Neoendothelialization on the left atrial surface of the device at 42 days after implantation
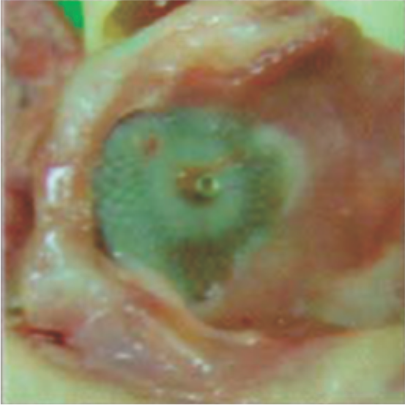
Neoendothelialization on the right atrial surface of the device at 36 days after implantation
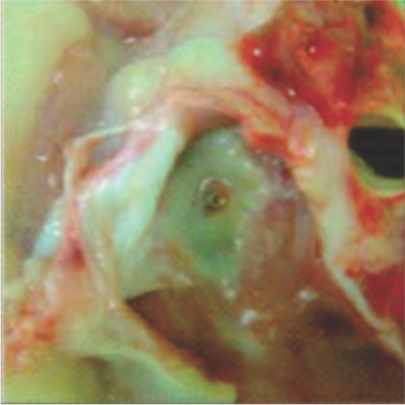
Neoendothelialization on the right atrial surface of the device at 42 days after implantation
ASD : Atrial Septal Defect
References:
1. Hellenic J Cardiol. 2021 May-Jun;62(3):206-211.
2. Medicine. 2019 Mar;98(10).
3. Int J Cardiol. 2014 Dec 15;177(2):418-22.
4. Catheter Cardiovasc Interv. 2015 Sep 1;86(3).
*Data on SMT file. R & D tests performed using 26 mm Septal Occluders for both devices. The test results are an average of the test performed three times.
#Recapture and redeployment is possible only if delivery cable is securely connected to the disc
Caution: This product is intended for use by or under the direction of a physician. Prior to use, refer to the "Instructions for use" supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.
Check the regulatory status of the device before distribution in areas where CE marking is not the regulation in force. Tests performed by and data on file at Sahajanand Medical Technologies Limited (SMT). Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photos on file at Sahajanand Medical Technologies Limited.
Cocoon Septal Occluder is manufactured by Vascular Innovations Co., Ltd. Thailand, a SMT group company. Cocoon is a trademark of Vascular Innovations Co., Ltd.
Cocoon range of occluders are currently not approved by USFDA and are not available for sale in USA.
Disclaimer: © 2025 Sahajanand Medical Technologies Limited – All rights reserved. Specifications are subject to modification, revision and improvement.



