
|
1-Year Outcomes from TUXEDO-II
Supraflex Cruz vs Xience in Patients with
Diabetes and MVD, Undergoing PCI
|
|
Key Findings:
Supraflex Cruz demonstrated comparable clinical outcomes as Xience with 23% numerically lower incidence
of target lesion revascularization in diabetic patients with multivessel disease (log-rank p = 0.44).
|
| |
Number of Patients:
1800
|
|
Geography:
63 sites
in India
|
|
| |
Study Chairman & Lead Principal Investigator:
Dr. Upendra Kaul, India
Study Co-Chair: Dr. Sripal Bangalore, USA
Primary Endpoint: TLF, composite of cardiac death, TV-MI and ischemia-driven
TLR, at one-year
|
|
|

|
1-Year Outcomes from Multivessel TALENT Trial
Supraflex Cruz vs Synergy Stent for Three-Vessel Coronary Artery Disease
|
|
Key Findings:
At 1-year, Supraflex Cruz demonstrated non-inferiority to Synergy, with a numerically lower rate of POCE Outcomes
(SYNTAX Score ≥ 33).
|
| |
Number of
Patients:
1550
|
|
Geography:
54 sites in Europe and the UK
|
|
| |
Chair & Chief Investigator: Prof. Patrick W. Serruys, Ireland
Principal Investigators: Prof. Helge Moellmann, Germany; Dr. Manel Sabate,
Spain; Prof. Azfar Zaman, UK
Primary Endpoint: POCE:composite of all-cause death, any stroke, any MI, and
any revascularization at 1-year
|
|
|

|
The FIRE Trial 3-Year Results
Supraflex Cruz in Elderly Patients with
Myocardial Infarction and Multivessel Disease
|
Key Findings:
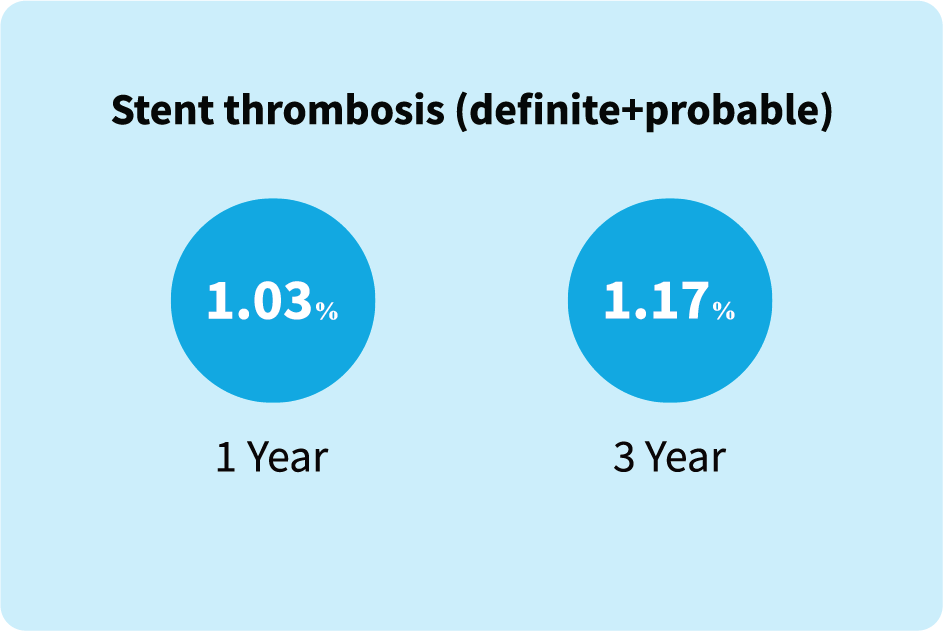
In a complex patient population (age ≥ 75 years) with MI and multivessel disease, Supraflex Cruz
continues to demonstrate extremely low rates of stent thrombosis (definite + probable) at long-term
follow-up of 3 years. |
 |
|
Number of
Patients:
1445 |
Geography:
34 sites
in Italy, Spain, and Poland |
Study Chair: Prof. Gianluca Campo, Italy
Principal Investigator: Dr. Simone Biscaglia, Italy
Primary Endpoint: Composite of death, myocardial infarction,
stroke, or ischemia-driven coronary revascularization
|
|

|
1-Year Outcomes in the First 5,000
Patients
Real-World Evaluation of Supraflex Family SES
|
|
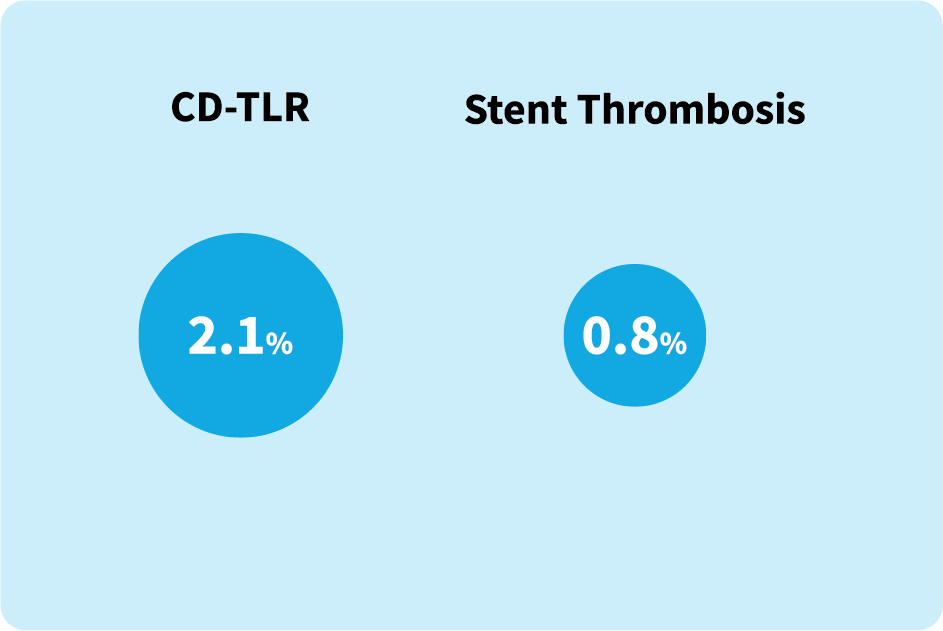
Key Findings:
Low incidence of clinically-driven target lesion revascularization and stent thrombosis at 1 year in
real-world patients.
|
|
|
Number of
Patients:
5000
|
Geography:
10 sites
in the Netherlands
|
Coordinating Investigator: Dr. Sander Ijsselmuiden
Primary Endpoint: TLF, composite of cardiac death, TV-MI, or
clinically driven TLR at 1-year
|
|

|
Supraflex Cruz in an Aged (≥80 years) Real-life Patients
Cohort representing the most complex high-risk patient cohort for PCI in daily routine
|
|
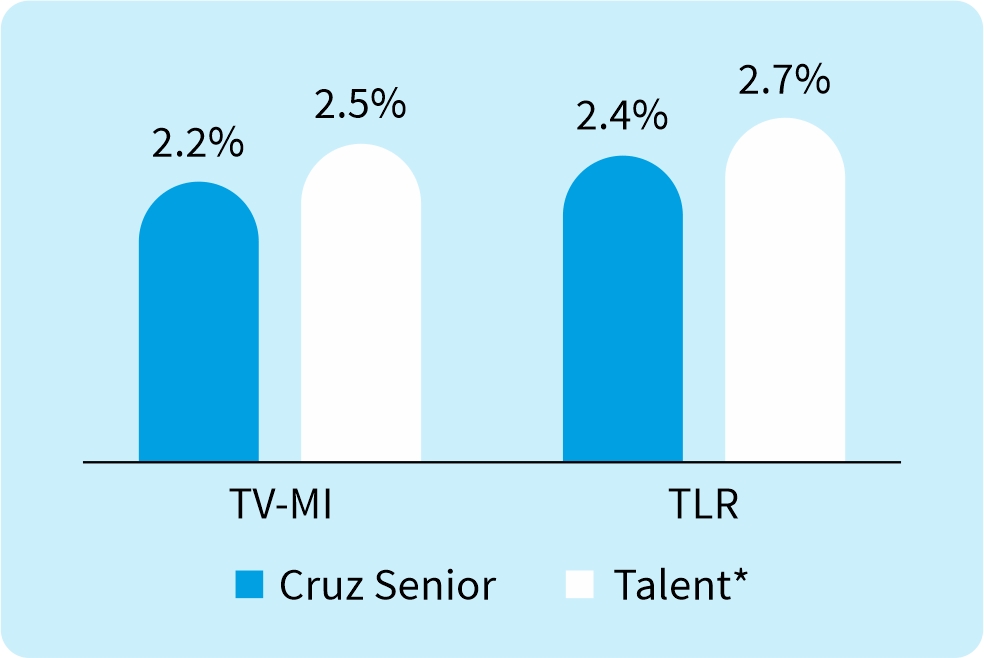
Key Findings:
The incidence of TLR and TV-MI with Supraflex Cruz in octogenarians and nonagenarians is comparable to outcomes observed in the regular all-comer PCI population.
|
|
|
Number of
Patients:
1993
|
Geography:
37 sites in Germany, Switzerland, Austria and France
|
Principal Investigator:Prof. David M. Leistner
Primary Endpoint:DOCE, composite of cardiovascular death, MI not clearly attributable to a non-target vessel, and clinically driven TLR
|
|
|
FOR MORE DETAILS PLEASE SCAN
|
TLF: Target Lesion Failure, TV-MI: Target Vessel Myocardial Infarction, TLR: Target Lesion
Revascularization, POCE: Patient-Oriented Composite Endpoint, MI: Myocardial Infarction, DOCE:
Device-Oriented Composite Endpoint, CABG: Coronary Artery Bypass Grafting, DES: Drug-Eluting Stent,
CD-TLR: Clinically-driven Target Lesion Revascularization
Disclaimer: Supraflex Cruz is a trademark of Sahajanand Medical Technologies Ltd. or its
affiliates. Synergy is a trademark of Boston Scientific Corporation or its affiliates. Xience is a
trademark of the Abbott Group of Companies. TCT is a trademark of Cardiovascular Research Foundation.
*Lancet. 2019 Mar 9;393(10175):987-997. doi: 10.1016/S0140-6736(18)32467-X. .
|
|

